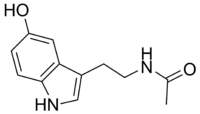
N-乙酰血清素(英语:N-Acetylserotonin,NAS,也作normelatonin)是一种天然存在的化合物,是从血清素到褪黑素的内源性合成的反应中间体[1][2]。它由血清素(又称为5-羟色胺)在N-乙酰基转移酶(AANAT)催化下与乙酰辅酶A反应产生,然后N-乙酰血清素再在乙酰血清素O-甲基转移酶(ASMT)催化下被S-腺苷甲硫氨酸甲基化为褪黑素。和褪黑素一样,N-乙酰血清素也是褪黑素受体(褪黑素受体1A、褪黑素受体1B和褪黑素受体1C)的激动剂,并且可以被认为是一种神经递质。[3][4][5][6]
最近,NAS已经显示出作为一种有效力的TrkB受体激动剂,而血清素和褪黑激素并没有此种机制。[3] 以"TrkB受体"为介导(TrkB-mediated)而产生出强劲的抗抑郁,神经保护(neuroprotection)和神经营养因子等效果。[3]
此外,光线照射抑制NAS的合成、和减少单胺氧化抑制剂的抗抑郁作用。[3] 这些数据强烈支持NAS在调节情绪和引起抗抑郁药的治疗效益之作用。
通过目前未知的机制,NAS可能是姿位性低血压的引发因子、且以"单胺氧化抑制剂"(MAOIs)作临床治疗。[7][8] It reduces blood pressure in rodents, and pinealectomy (the pineal gland being a major site of NAS and melatonin synthesis) abolishes the hypotensive effects of clorgyline.[7][8] 为什么"姿位性低血压"常见与"单胺氧化抑制剂"(MAOIs)一起发生,而不与SSRIs(这两者均增加NAS级别)一起,而这方面并不清楚。
另见
参考文献
- ↑ AXELROD J, WEISSBACH H. Enzymatic O-methylation of N-acetylserotonin to melatonin. Science. April 1960, 131 (3409): 1312. doi:10.1126/science.131.3409.1312. PMID 13795316.
- ↑ WEISSBACH H, REDFIELD BG, AXELROD J. Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. Biochimica et Biophysica Acta. September 1960, 43: 352–3. doi:10.1016/0006-3002(60)90453-4. PMID 13784117.
- ↑ 3.0 3.1 3.2 3.3 Jang SW, Liu X, Pradoldej S, et al.. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proceedings of the National Academy of Sciences of the United States of America. February 2010, 107 (8): 3876. doi:10.1073/pnas.0912531107. PMID 20133677. PMC 2840510.
- ↑ Zhao H, Poon AM, Pang SF. Pharmacological characterization, molecular subtyping, and autoradiographic localization of putative melatonin receptors in uterine endometrium of estrous rats. Life Sciences. March 2000, 66 (17): 1581–91. doi:10.1016/S0024-3205(00)00478-1. PMID 11261588.
- ↑ Nonno R, Pannacci M, Lucini V, Angeloni D, Fraschini F, Stankov BM. [http//www.ncbi.nlm.nih.gov/pmc/articles/PMC1566130/ Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists]. British Journal of Pharmacology. July 1999, 127 (5): 1288–94. doi:10.1038/sj.bjp.0702658. PMID 10455277. PMC 1566130.
- ↑ Paul P, Lahaye C, Delagrange P, Nicolas JP, Canet E, Boutin JA. Characterization of 2-[125Iiodomelatonin binding sites in Syrian hamster peripheral organs]. The Journal of Pharmacology and Experimental Therapeutics. July 1999, 290 (1): 334–40. PMID 10381796.
- ↑ 7.0 7.1 Oxenkrug GF. [N-acetylserotonin and hypotensive effect of MAO-A inhibitors]. Voprosy Meditsinskoi Khimii. 1997, 43 (6): 522–6. PMID 9503569 (Russian).
- ↑ 引用错误:无效
<ref>标签;未为name属性为pmid10591054的引用提供文字
|
|
|
| 直接衍生物 |
|
|
氨基N-取代
衍生物 |
|
|
氨基N,N-二取代
衍生物 |
|
|
参考来源
更多医学百科条目