诺维乔克
| A+医学百科 >> 诺维乔克 |
诺维乔克(俄文новичок,Novichok)是苏联在1970年代及1980年代开发的一系列神经毒剂[1]。据称有些衍生物的毒性可能是 VX (神经毒剂)的五至八倍,但无法得到证实[2][3]。诺维乔克属于苏联“Foliant”计划中开发的第四代化学武器[4],最早的名称是K-84,后来更名为A-230。诺维乔克系列包括超过一百种不同结构的变体[5],其中最有军事价值[来源请求]的是A-232(Novichok-5;[(2-chloro-1-methylethoxy)fluorohydroxy phosphinyl]oxy]carbonimidic chloride fluoride];氟磷酸氟氯二取代亚甲胺基(2-氯)异丙基酯),其次是A-230(Novichok-7;(2-chloroethoxy)fluorohydroxyphosphinyl]oxy] carbonimidic chloride fluoride;氟磷酸氟氯二取代亚甲胺基(2-氯)乙基酯)。
在1980年苏联化学家米尔査扬诺夫在《国家的秘密:俄罗斯化学武器计划内部知情人士的记述》一书中公开诺维乔克结构。[来源请求]Steven L. Hoenig在《化学与生物战剂手册》第二版中亦列出了部分Novichok家族成员的分子结构。
化学结构
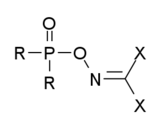
有二种有机磷制剂都被归类为诺维乔克毒剂。第一种是有dihaloformaldoxime基团的有机磷化合物,其通式如下:其中的R 可以是烷基、烷氧基、烷氨基或氟,而X为卤素(氟、氯、溴)或是像C≡N等拟卤素。这些化合物被广泛地记载在当时的苏联文献中,但不确定是否包括所有的诺维乔克毒剂[6][7][8][9][10][11][12][13]。
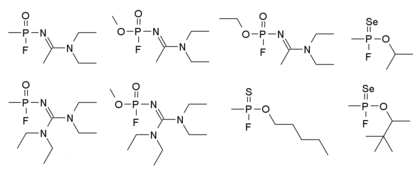
Mirzayanov在自传中有提到另一种结构的诺维乔克诺维乔克毒剂,其结构如下:他明确的制作了大量的化合物,许多毒性较低的衍生物也在公开文献中以新型有机磷杀虫剂的方式提及,因此秘密的化学武器研究可以伪装为合法的农药研究[14]。
参考资料
- ↑ Tucker, J. B.; War of Nerves; Anchor Books; New York; 2006; pp 232-233.
- ↑ Vadim J. Birstein. The Perversion Of Knowledge: The True Story of Soviet Science. Westview Press (2004) ISBN 0-8133-4280-5
- ↑ Yevgenia Albats and Catherine A. Fitzpatrick. The State Within a State: The KGB and Its Hold on Russia — Past, Present, and Future, 1994. ISBN 0-374-18104-7 (see pages 325-328)
- ↑ Tucker, J. B.; War of Nerves; Anchor Books; New York; 2006; pp 231.
- ↑ Tucker, J. B.; War of Nerves; Anchor Books; New York; 2006; pp 233.
- ↑ Kruglyak Yu L, Malekin SI, Martynov IV. Phosphorylated oximes. XII. Reactions of 2-halophospholanes with dichlorofluoronitrosomethane. Zhurnal Obshchei Khimii. 1972; 42(4):811-14.
- ↑ Raevskii OA, Chapysheva NV, Ivanov AN, Sokolov VB, Martynov IV. Effect of Alkyl Substituents in Phosphorylated Oximes. Zhurnal Obshchei Khimii. 1987; 57(12):2720-2723
- ↑ Raevskii OA, Grigor'ev V Yu, Solov'ev VP, Ivanov AN, Sokolov VB, Martynov IV. Electron-Donor Functions of Ethyl Methylchloroformimino Methylphosphonate. Zhurnal Obshchei Khimii. 1987; 57(9):2073-2078
- ↑ Makhaeva GF, Filonenko IV, Yankovskaya VL, Fomicheva SB, Malygin VV. Comparative studies of O,O-dialkyl-O-chloromethylchloroformimino phosphates: interaction with neuropathy target esterase and acetylcholinesterase. Neurotoxicology. 1998 Aug-Oct;19(4-5):623-8. PMID 9745921
- ↑ Raevskiĭ OA, Chistiakov VV, Agabekian RS, Sapegin AM, Zefirov NS. Formation of models of the interaction between organophosphate compound structure and their ability to inhibit cholinesterase. Bioorganicheskaia Khimiia. 1990 Nov;16(11):1509-22. PMID 2096825
- ↑ Ivanov IuIa, Sokolov VB, Epishina TA, Martynov IV. O-substituted alkylchloroformoximes as substrates and inhibitors of cholinesterases. Doklady Akademii Nauk SSSR. 1990;310(5):1253-5. PMID 2354654
- ↑ Malygin VV, Sokolov VB, Richardson RJ, Makhaeva GF. Quantitative structure-activity relationships predict the delayed neurotoxicity potential of a series of O-alkyl-O-methylchloroformimino phenylphosphonates. Journal of Toxicology and Environmental Health Part A. 2003 Apr 11;66(7):611-25. PMID 12746136
- ↑ Steven L. Hoenig. Compendium of Chemical Warfare Agents. Springer New York, 2007. ISBN 978-0-387-34626-7
- ↑ Vil S Mirzayanov. State Secrets. An Insider's Chronicle of the Russian Chemical Weapons Program. (2009) pp142-145, 179-180. ISBN 978-1-4327-2566-2
|
|||||||||||||||||||||||||||||||||||||||||||||||
参考来源
| 关于“诺维乔克”的留言: | |
|
目前暂无留言 | |
| 添加留言 | |